Generating an experiment ID report
The software automatically generates an experiment ID report when the experiment ID is closed. The following procedure describes how to generate a report for any experiment ID selected in the Experiments Editor.
Creating a report for a selected experiment ID
To create an experiment ID report
1 Open the Experiments Editor using one of the following methods:
• In a VWorks form, click the Experiments Editor or Select Experiment button.
• In the VWorks window, click Tools->Experiments Editor.
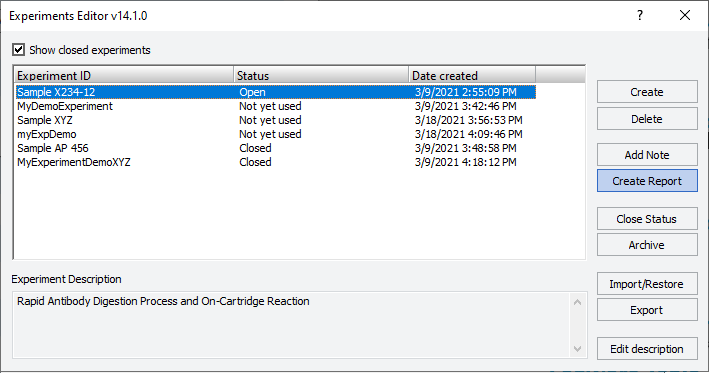
2 Optional. To include closed experiment IDs in the Experiments Editor window, select the Show closed experiments check box.
3 Select the Experiment ID and then click Create Report.
 |
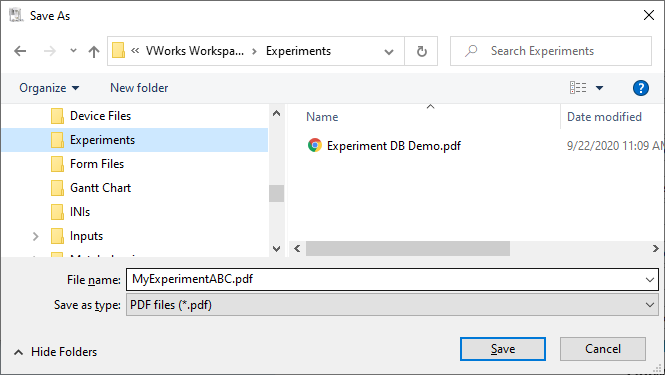
4 In the Save As dialog box, type a file name for the report, select a storage location, and then click Save. The software saves the report as a pdf file.
 |
Note: The VWorks software uses the Microsoft Print to PDF virtual printer to generate .pdf files. This virtual printer is installed on all Windows 10 and 11 systems by default. If the virtual print has been removed, the report generation feature will not work.
Experiment ID report contents
The following figure shows an example of an experiment ID report, the following table describes the report contents.
Figure Example experiment ID report
 |
Table Report contents
Item | Description |
|---|---|
Report header | Date that the report was generated • Experiment ID, creation date, and user log in of experiment ID creator • Description provided for the experiment |
Archival status and history, if applicable | Archival status of experiment ID: • Date of archival or restoration • User who archived or restored • File name and path of the archived to or restored from location |
Application and utility details | • Application or utility name • Iteration of the protocol run, and if it was simulated. Each iteration is listed separately, and ordered by launch time. Individual steps within an application or utility iteration are separated by a single blank line. • Log in of the user • Date and time run was started • Method name • Number of full columns of cartridges • Labware selection for each deck location • Step details for each step in the application |
Notes | • Date and time the note was appended and the user login • Note contents |