Compliance-enabling features
The combination of features in VWorks Plus 14.1.1.1 and Protein Sample Prep Workbench 4.0 help enable compliance with Part 11 of Title 21 of the Code of Federal Regulations (21 CFR Part 11). The FDA rules and guidelines for compliant electronic records and computerized systems require secure data handling, including:
• Data security. Physical protection of data by limiting access to the system and preventing unauthorized access.
• Data integrity. Protecting raw data and metadata and preventing these from unauthorized modification, and linking raw data and results to reproduce the original results at any time, for example, in an audit situation, and document each new result copy.
• Audit traceability. Documenting who did what to the results and when.
VWorks Plus features that support compliance
VWorks Plus compliance-enabling features include user authentication, content management, tamper detection, audit trails, electronic signatures, and activity logs. For a description of these features, see the Using compliance features chapter in the VWorks Automation Control Setup Guide.
Workbench features that support compliance
The Protein Sample Prep Workbench uses experiment IDs and methods to support traceability and data integrity. An experiment ID and a method are required to run any application or utility in the compliance-enabled Protein Sample Prep Workbench.
• An experiment ID is a database record that captures the steps executed and the settings used during each run of an application or utility. Any errors that may have occurred during a run are also recorded.
Electronic signatures are required to close an experiment ID. The software automatically generates a report when an experiment ID is closed. A report describes who did what and when for a given experiment ID.
For details, see Using Experiment IDs.
• A method is a comprehensive collection of saved settings for an application or utility that you can use to run the application or utility.
Methods in the OpenLab Content Management storage can be used to run an app or utility protocol.
Any changes to an existing method must be saved using a new name before the revised method can be used to run an app or utility protocol.
For detailed procedures, see the user guide for the app or utility.
Roles and privileges
The VWorks administrator, technician, and operator roles have the following privileges in the compliance-enabled Workbench.
VWorks role | Privileges for experiment IDs and methods |
|---|---|
VWorks operator | Experiment IDs • Select an experiment ID to run an app or utility • Add notes to an experiment ID • Generate an experiment ID report • Export experiment IDs Methods • Select a method to run an app or utility • Export a method |
VWorks technician | All the privileges of a VWorks operator, plus the following: Experiment IDs • Create experiment IDs and edit the description of experiment IDs that have the Not Yet Used status • Close experiment IDs and post a signature when closing • Import experiment IDs Methods • Create and save methods • Import methods |
VWorks administrator | All the privileges of a VWorks operator and technician, plus the following: • Archive closed experiment IDs • Delete experiment IDs that have a Not Yet Used status • Log in to Content Browser and edit the project, including deleting files |
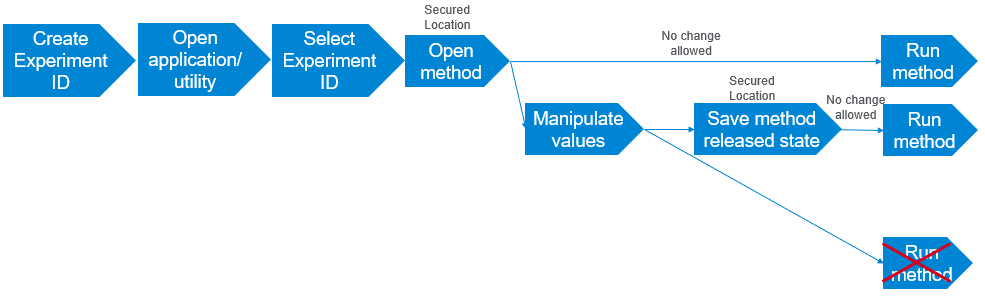
Figure VWorks Technician privileges for creating and running methods
 |