Preparing the samples

When preparing the samples, you must:
• Remove macromolecular particulates before the samples are loaded onto AssayMAP cartridges.
• Adjust the buffer composition to the optimal binding conditions (for example, low pH and low organic).
• Determine the volume of samples to load on the AssayMAP cartridges.
• Transfer the samples to the microplate you want to use for the protocol run.
Removing macromolecular particulates
Make sure the samples are free of macromolecular particulates, such as large protein aggregates and cellular debris to prevent clogging the cartridges. Samples should be filtered through a 0.45‑µm filter or centrifuged at a high g‑force before loading on an AssayMAP cartridge.
Adjusting the buffer composition
One of the most important considerations for highly efficient and unbiased binding of peptides to the reversed-phase resin is the pH of the sample, which should be acidic for standard, low-pH peptide cleanup with C18 or RP-S cartridges or basic with RP-S cartridges.
Peptides are amphoteric molecules with a diverse range of physiochemical properties. For some classes of peptides, cleanup under high-pH conditions may help to promote retention (e.g., highly basic peptides), or preserve acid-labile modifications (e.g., histidine phosphorylation). You can acidify samples for low-pH peptide cleanup before loading onto AssayMAP C18 and RP-S cartridges generally by adding TFA, formic acid, or acetic acid. Sample pH for high-pH peptide cleanup is commonly adjusted using aqueous solutions of ammonium formate or ammonium acetate titrated to a pH > 10 using ammonium hydroxide. You can use the Reagent Transfer utility to perform these pH adjustments. For instructions, see Reagent Transfer v3.0 User Guide.
Samples containing organic solvents or some types of detergents should be avoided as they might bias the peptides that bind the column. For example, loading samples in a buffer containing greater than 5% acetonitrile will inhibit binding of hydrophilic peptides. Silica-based C18 cartridges are at risk with a sample pH higher than 8. If you have concerns about a specific buffer component, you should examine scientific literature for the known effects of this type of molecule on reversed-phase resins. You can use the Reagent Transfer utility to modify the composition of your samples with a dilution or pH adjustment, especially after using the In-Solution Digestion workflow.
Determining the volume of sample to load
The AssayMAP Peptide Cleanup protocol permits loading up to 1000 µL of sample onto AssayMAP cartridges. For sample volumes > 250 µL, the protocol will iteratively load samples onto cartridges to stay within the maximum syringe volume (250 µL) of the Bravo 96AM Head.
What is the binding capacity of the cartridge?
Two ways to express the binding capacity of a cartridge are quantitative binding capacity and total binding capacity:
• Quantitative binding capacity. The maximum mass of peptide that can bind to the cartridge in a single pass, where less than 10% of the load appears in the flow-through.
For a single species of peptide, the quantitative binding capacity is relatively straightforward. The quantitative binding capacity for a mixture of peptides is more complex due to the differences in relative hydrophobicity of the peptides, which results in competitive binding in situations where the ratio of binding sites to mass loaded is low.
Examples of how sample mass loaded and the relative hydrophobicity of peptides can affect recovery in a complex peptide mixture, see Agilent app note 5991-2957EN in the Reference library. To avoid or minimize sample bias during peptide cleanup, it is critical to load sample masses that are less than the mass at which hydrophillic peptides are lost.
• Total binding capacity. The maximum mass of peptide that can bind to the cartridge. This can only be achieved by loading significantly more peptide than can be bound by the cartridge. This value is significantly greater than the quantitative binding capacity and will result in the loss of hydrophillic peptides.
See the Agilent AssayMAP Bravo Cartridges Selection Guide for detailed information about the binding capacity for the 5 and 25 µL RPS and C18 cartridges.
What is the concentration of the target in the sample?
If you know the approximate concentration of the target molecule in your sample and you are working within the quantitative binding range of the cartridge, you can determine the volume of sample to load as follows:
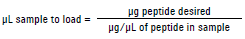 |
Preparing the sample plates
Planning the microplate setup
Before transferring the samples, you should plan the layout of the samples in the microplate. Consider the following:
• You can process 1 to 96 samples in parallel. The position of the samples in the microplate dictates the positions of the cartridges in the 96AM Cartridge & Tip Seating Station. These positions must also match the locations of the buffer solutions in microplates and reservoirs.
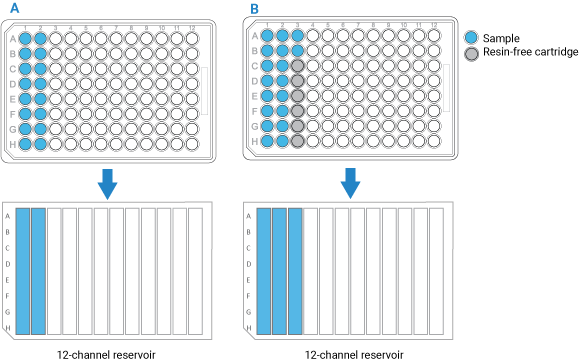
• If you have fewer than 96 samples, make sure the samples occupy full columns in the microplate, as the figure below shows.
• The default protocol settings assume that samples will be arranged in multiples of 8 in a column-based configuration. Also, the Bravo Platform applies differential pressure to seat cartridges based upon the number of full columns of cartridges. To achieve proper cartridge seating, entire columns must be used.
• If the number of samples you have is not a multiple of 8, use the AssayMAP Resin-Free cartridges (Agilent part number G5496-60009) to fill the empty column and row positions. This will prevent liquids from dripping on the deck or being dispensed on the deck during the Cup Wash step.
Figure Example of sample microplate and reservoir layout: A) Multiple of 8 samples, B) Not a multiple of 8
 |
See Labware for acceptable labware at each deck location.
Transferring the samples to the microplate
An excess (overage) volume ensures that a microplate well or column does not fully deplete, which would result in aspiration of air into the syringes and then into the cartridges, compromising performance.
The Reagent Volume Calculator shows the recommended overage for the labware types being used and automatically includes recommended overages in the volume it recommends per well.
Labware-specific overage recommendations are also presented in the Labware Reference Guide, which you can find in the Literature Library page of the Protein Sample Prep Workbench. More or less overage can be used depending on the volatility of the solution and the length of the run but the recommended overages are fine for most standard runs.
To transfer the samples to the microplate:
1 Run the Reagent Transfer utility or Reformatting utility to transfer the samples. For instructions, see one of the following:
2 If necessary, centrifuge the sample labware to remove bubbles.