Preparing the solutions

The following solutions are required for the Phosphopeptide Enrichment protocol:
• Priming Buffer and Syringe Wash Buffer
• Equilibration and Cartridge Wash Buffer
• Elution Buffer
Note: You can find the Labware Reference Guide in the Literature Library page of the Protein Sample Prep Workbench.
Using the Reagent Volume Calculator for Phosphopeptide Enrichment
The Reagent Volume Calculator is a Microsoft Excel file that contains a Calculator worksheet. You enter the number of columns to process, whether to perform the Collect Flow Through options, the volume for each step in the protocol, the number of wash cycles to conduct, and the labware selection for each deck location. The calculator determines the volumes required based on your input, taking into consideration pipetting overage and evaporation concerns.
Note: The pipetting overage suggested is generally conservative. The minimal overage may be greater or less depending on the volatility of the solution, the length of the run, and when the step occurs during the run. The overage volume can be optimized to minimize loss of precious reagents.
To use the Reagent Volume Calculator:
1 Open the App Library.
2 Locate the application, and then click the corresponding Calculator button. Microsoft Excel starts and displays the calculator.
3 Ensure that you enable content in Microsoft Excel.
4 Click one of the following:
• Set defaults for 5µL cartridges. Sets the values in the calculator using the values from the default method for the 5 µL cartridges.
• Set defaults for 25µL cartridges. Sets the values in the calculator using the values from the default method for the 25 µL cartridges.
5 Modify the values in the green boxes as required to match your specific method. As you change the values in the green boxes, the calculated values change.
Note: The green box should remain green after you enter a value. If you enter a value that is outside the normal working range, the box becomes yellow. If you enter a value that is outside of the acceptable range, the box becomes red.
To display the corresponding tooltip for a setting, mouse over a box that has a red triangle in the upper right corner.
The following figure shows the Reagent Volume Calculator.
Figure Phosphopeptide Enrichment Calculator worksheet
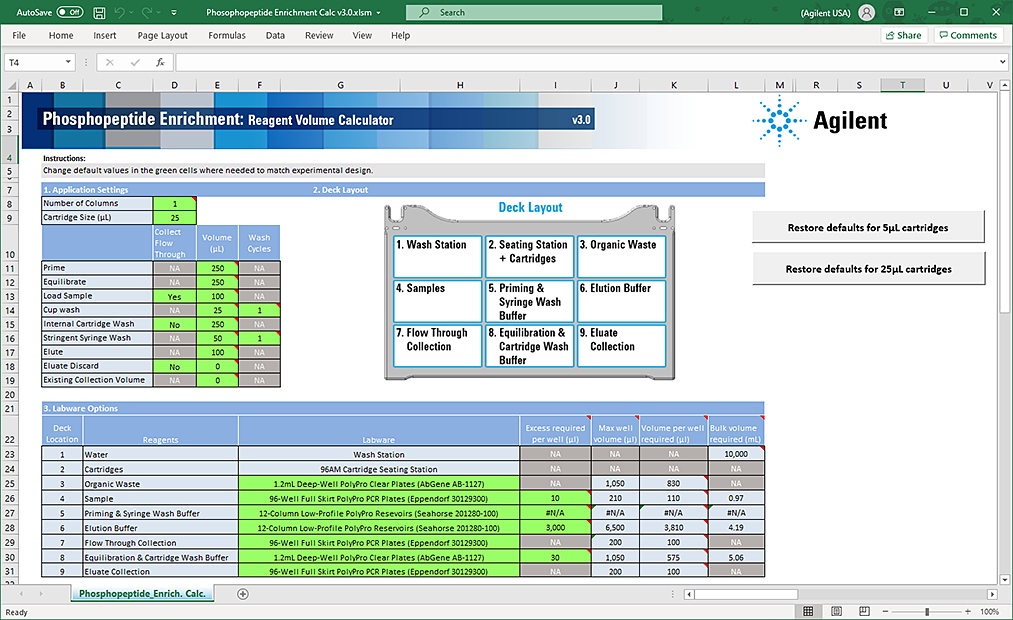 |
Preparing the buffers
The following table describes the reagents and deck locations. The AssayMAP protocols are blind to the composition of the solutions, so you can easily adapt your optimized chemistry. Agilent recommends the following buffers as a starting point for optimizing the AssayMAP phosphopeptide enrichment chemistry.
Table Reagent preparation
Reagent (deck location) | Composition and comments |
|---|---|
Priming Buffer & Syringe Wash Buffer (deck location 5) | This dual-purpose buffer serves as both the Priming Buffer and Syringe Wash Buffer during the protocol run. Do not alter the composition without evaluating how the change affects the priming step. Organic solvent in the buffer helps to purge entrained air within the cartridge resin bed during priming and is effective at removing hydrophobic species from syringes as part of the stringent syringe wash. Priming with a high-pH buffer helps to prepare TiO2 cartridges better than priming with a low-pH buffer. The following buffers have been used successfully with the indicated cartridges. Fe(III)-NTA: 99.9% ACN : 0.1% TFA TiO2 : 50% ACN : 45% H2O : 5% NH3 |
Elution Buffer (deck location 6) | Dilute solutions of aqueous ammonia are sufficient to elute phosphopeptides from Fe(III)-NTA and TiO2 cartridges. Recovery of hydrophobic peptides may be improved with the addition of small percentages of acetonitrile. The following buffers have been successfully used with the indicated cartridges. Fe(III)-NTA: 99 % H2O : 1% NH3 TiO2: 80 % H2O : 15% ACN : 5% NH3 |
Equilibration & Cartridge Wash Buffer (deck location 8) | This solution should be similar in composition to the sample loading buffer. The following buffers have been successfully used with the indicated cartridges. Fe(III)-NTA: 80% ACN : 19.9% H2O : 0.1% TFA TiO2: 50% ACN : 48 % H2O : 2% TFA |
Note: All suggested solutions listed as percentages are volume/volume formulations.
Note: The source of NH3 for these buffers is aqueous ammonium hydroxide, which is commonly listed as %NH3 in H2O from commercial suppliers.
Dispensing the solutions
To dispense the solutions into the labware:
1 Optional. Label the labware so that you can easily identify them.
2 Add the specified volume of Priming & Syringe Wash Buffer into the labware to be placed at deck location 5.
3 Add the specified volume of Elution Buffer into the labware to be placed at deck location 6.
4 Add the specified volume of Equilibration & Cartridge Wash Buffer into the labware to be placed at deck location 8.
5 If necessary, centrifuge the reagent labware to remove bubbles.
Note: You can use the Reagent Aliquot utility to dispense the buffers. For details, see Reagent Aliquot v2.0 User Guide.