Preparing the samples

When preparing the samples, you must:
• Remove macromolecular particulates before the samples are loaded onto AssayMAP cartridges.
• Adjust the buffer composition to optimize the binding conditions.
• Determine the volume of samples to load on the AssayMAP cartridges.
• Transfer the samples to the microplate you want to use for the protocol run.
Removing macromolecular particulates
Make sure the samples are free of macromolecular particulates, such as large protein aggregates and cellular debris to prevent clogging the cartridges. Samples should be filtered through a 0.45‑µm filter or centrifuged at a high g‑force before loading on an AssayMAP cartridge.
Adjusting the sample composition
Optimal conditions for phosphopeptide binding to AssayMAP phosphopeptide enrichment cartridges
In a review of relevant literature for phosphopeptide enrichment using various titanium dioxide formats (2–28) and immobilized metal affinity chromatography (IMAC) formats (8, 29–55), there was no clear consensus on sample and reagent formulations that yielded the optimum phosphopeptide enrichment ratio, the highest absolute phosphopeptide recovery, and the greatest reproducibility. In the majority of these studies, reagent formulations were optimized for the experiment at hand.
Similarly, phosphopeptide enrichment using AssayMAP Fe(III)-NTA and TiO2 cartridges requires some degree of chemistry optimization and method development depending on the desired analytical result. No one set of chemistry conditions will be universally optimal. However, there are some chemistry trends that can serve as reasonable starting points for method development.
Typically, samples for phosphopeptide enrichment using Fe(III)-NTA and TiO2 have first been subjected to a reversed-phase cleanup (desalting) step. Samples are generally in a phosphate-free, TFA-containing, low-pH buffer (pH 2.4 to 2.8) with some amount of organic solvent. Low-pH conditions reduce non-specific binding of non-phosphopeptides by facilitating protonation of acidic side-chains and C-termini of peptides, whereas the phosphoryl groups of phosphopeptides remain negatively charged at low pH to promote binding in a combination of ion exchange and metal coordination to the stationary phase (2, 56). The presence of organic solvent (typically 25% to 80% acetonitrile) in the sample and wash solutions helps to reduce nonspecific binding of non-phosphopeptides. Organic solvent serves to modulate the pKa values of ionizable species and the effect can be very different depending on the chemical species (57, 58). Achieving high phosphopeptide enrichment often means finding conditions that maximize the difference in the pKa values of phosphoryl and carboxyl groups such that under a given set of solvent conditions, the phosphoryl groups are negatively charged while carboxyl groups are neutral (45, 49). This is accomplished by adjusting the type and concentration of both the acid modifier and organic solvent present in the samples and wash buffers. Specificity can also be improved for phosphopeptide enrichment by TiO2 with the addition of small-molecule organic acids (such as, glycolic acid, lactic acid, and dihydroxybenzoic acid), which may further reduce nonspecific binding of non-phosphopeptides by competitively displacing weakly bound acidic, non-phosphopeptides.
Sample components that cause concerns
Samples should be free of phosphates, free of particulates, and contain no or low concentrations of salts, denaturants, and chaotropes. The best results are typically achieved using samples first subjected to reversed-phase cleanup (desalting) before phosphopeptide enrichment. Solubilization of peptide samples after desalting and drying can be problematic when using high levels of organic solvent in the sample solubilization or reconstitution buffer. Solubility is often promoted by first adding the aqueous component of the buffer to the sample along with thorough mixing. This is followed by a slow titration of the organic portion of the buffer into the sample until the desired aqueous or organic ratio is achieved.
Poor enrichment efficiency can be caused by insoluble or precipitated peptides that can form particulates large enough to collect at the top of the resin bed in the cartridge during sample loading. These particulates will mostly likely contain a very high percentage of non-phosphopeptides. If the wash buffer is insufficient to solubilize the particulates collected at the top of the cartridge, it is likely that the high-pH, aqueous conditions used for elution will solubilize the particulates. The peptides from the particulates will co-elute with the phosphopeptides and decrease the percent enrichment. If sample particulates form post filtration, attempt to refilter the sample or pellet the particulates by centrifugation before sample loading.
Sample load volume and cartridge capacity
The AssayMAP Phosphopeptide Enrichment protocol permits loading of up to 1000-µL sample volumes onto AssayMAP phosphopeptide enrichment cartridges. For sample volumes > 250 µL, the protocol will iteratively load samples onto cartridges to stay within the maximum AssayMAP syringe volume (250 µL).
For each AssayMAP Fe(III)-NTA and TiO2 cartridge, the quantitative binding capacity (≥ 90% recovery) depends on the chemical characteristics of the phosphopeptide targets and the sample matrix. Matrix effects include the overall sample complexity and the presence and abundance of certain of solvents, buffers components, additives, and the pH of the sample.
For a tryptic digest of bovine a-casein, ~10 mole phosphate per mole protein (59), quantitative recovery of phosphopeptides is achieved with up to 1200-µg digest (~500 nmol phosphate) loaded onto TiO2 cartridges and up to 150-µg digest (~ 65 nmol phosphate) loaded onto Fe(III)-NTA cartridges.
AssayMAP Fe(III)-NTA cartridges have an iron content greater than 100 nmol Fe(III) per cartridge as determined by inductively coupled plasma—optical emission spectroscopy (ICP-OES).
For enrichment of the small molecule test substrate phenylphosphate (4, 27), the cartridge capacity at saturation is approximately:
• 115 µg (~ 660 nmol) per TiO2 cartridge
• 16 µg (~ 92 nmol) per Fe(III)-NTA cartridge
Preparing the sample plates
Planning the microplate setup
Before transferring the samples, you should plan the layout of the samples in the microplate. Consider the following:
• You can process 1 to 96 samples in parallel. The position of the samples in the microplate dictates the positions of the cartridges in the 96AM Cartridge & Tip Seating Station. These positions must also match the locations of the buffer solutions in microplates and reservoirs.
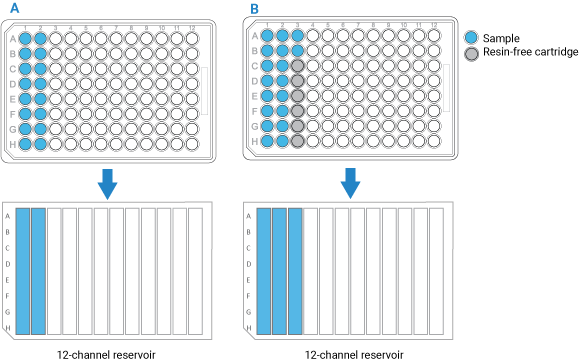
• If you have fewer than 96 samples, make sure the samples occupy full columns in the microplate, as the figure below shows.
• The default protocol settings assume that samples will be arranged in multiples of 8 in a column-based configuration. Also, the AssayMAP Bravo Platform applies differential pressure to seat cartridges based upon the number of full columns of cartridges. To achieve proper cartridge seating, entire columns must be used.
• If the number of samples you have is not a multiple of 8, use the AssayMAP Resin-Free cartridges to fill the empty column and row positions. This will prevent liquids from dripping on the deck or being dispensed on the deck during the Cup Wash step.
Figure Example of sample microplate and reservoir layout: A) Multiple of 8 samples, B) Not a multiple of 8
 |
See Labware for acceptable labware at each deck location.
Transferring the samples to the microplate
An excess (overage) volume ensures that a microplate well does not fully deplete, which would result in aspiration of air into the syringes and then into the cartridges, compromising performance.
The Reagent Volume Calculator shows the recommended overage for the labware types being used and automatically includes recommended overages in the volume it recommends per well. See Using the Reagent Volume Calculator for Phosphopeptide Enrichment.
Labware-specific overage recommendations are also presented in the Labware Reference Guide, which you can find in the Literature Library page of the Protein Sample Prep Workbench. More or less overage can be used depending on the volatility of the solution and the length of the run but the recommended overages are fine for most standard runs.
To transfer the samples to the microplate:
1 Run the Reagent Transfer utility or Reformatting utility to transfer the samples. For instructions, see one of the following:
2 If necessary, centrifuge the sample labware to remove bubbles.