Setting up a Normalization method

The Method Setup Tool is a Microsoft Excel-based tool that steps you through the process of creating and saving a method file that contains all the information required to run the Normalization protocol on the AssayMAP Bravo Platform. This tool uses formulas to calculate volume requirements for the samples and diluent based on your input.
Note: When you select a method in the Normalization utility, the form displays the corresponding labware selections and diluent preparation instructions.
Figure Normalization Method Setup Tool
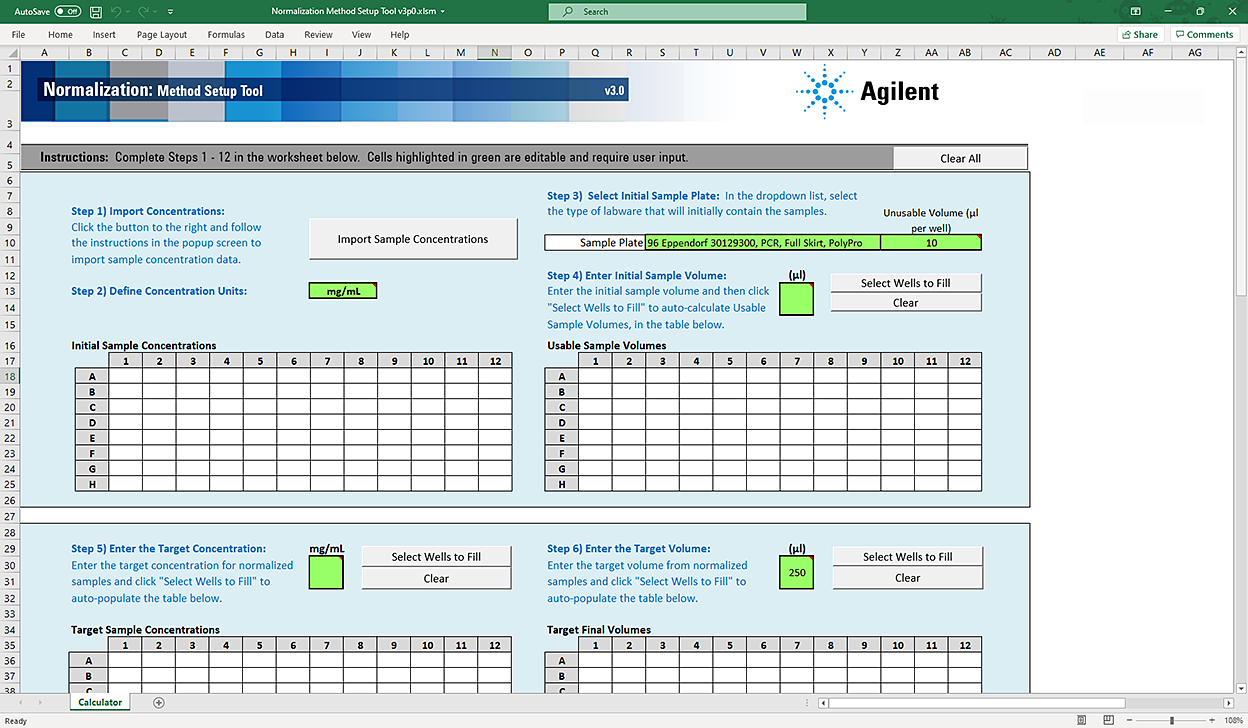 |
Before you start
Ensure that you have a Microsoft Excel file or a delimited text file (.csv or .txt) that contains the sample concentration values to be imported. The file should contain an 8‑by‑12 array of values, where
• Each row of the 8‑by‑12 array represents a row in the 96-well microplate.
• Each column of the 8‑by‑12 array represents a column in the 96-well microplate.
• An empty cell or a value of 0 is acceptable.
Figure Example of an Excel file with an 8-by-12 grid numbers
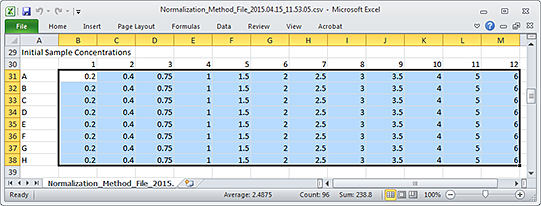 |
Figure Example of a csv file with an 8-by-12 grid numbers
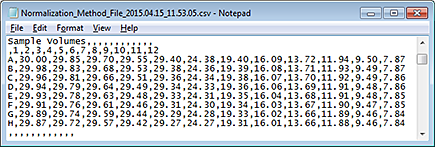 |
Opening the Method Setup Tool
You can open the tool from the Utility Library or from the Normalization form.
To open the Method Setup Tool:
1 In the Utility Library, locate the Normalization banner.
 |
2 Click one of the following buttons:
• Method Setup Tool. Microsoft Excel starts and displays the Method Setup Tool.
• Utility. The Normalization utility opens.
In the navigation pane, click Method Setup Tool. Microsoft Excel starts and displays the Method Setup Tool.
 |
Overview of steps in Method Setup Tool
The Method Setup Tool has 13 distinct steps. The following table provides an overview of the steps.
Table Overview of steps in Method Setup Tool
Step | Description | |
|---|---|---|
Defining Initial Samples plate (steps 1–4) | ||
1 | Import concentrations | Click Import Sample Concentrations, and then import the values for the known concentrations in your sample plate from a file (.xls, .xlsx, .csv, or .txt) or enter the values manually. |
2 | Define Concentration Units | Enter the concentration units in the green box. Note: This input is only a reminder. It has no impact on the subsequent calculations. |
3 | Select Initial Samples Plate | Click the green Sample Plate box, and then select the labware from the list. The setup tool uses the value that appears in the Unusable Volume box (also known as dead volume) to calculate the usable sample volume. You can change the unusable volume manually, if desired, as the default value is a conservative estimate. |
4 | Enter Initial Sample Volume | Type the initial sample volume in the green (µL) box, and then select the array of wells for which this value is correct in the Usable Sample Volumes plate grid. The setup tool automatically enters the usable sample volume (that is, the initial sample volume minus the unusable volume) in the Usable Sample Volumes plate grid. Repeat step 4 until you have specified the volume for all the wells in the Sample plate that contain a sample. |
Defining normalization targets (steps 5–6) | ||
5 | Enter the Target Concentrations | Type the target concentration in the green box, click Select Wells to Fill, and then select the array of wells to be filled in the Target Sample Concentrations plate grid. Repeat step 5 until you have specified the concentrations for all the wells. |
6 | Enter the Target Volume | Type a value in the green (µL) box that you want to apply to multiple wells of the microplate, and then click Select Wells to Fill. Select the array of wells to be filled in the Target Final Volumes plate grid. Repeat step 6 until you have specified the volumes for all the wells. |
Calculating results and dealing with exceptions (steps 7–8) | ||
7 | Calculate Normalization Volumes | Click Calculate Volumes. The calculated volumes (µL) display in the Diluent Volumes to Use and the Sample Volumes to Use plate grids. |
8 | Manage Calculation Exceptions | Click Check Exceptions. In the Manage Exceptions dialog box, select the option that is appropriate for each sample that is flagged as problematic. The setup tool wizard guides you through the process. |
Finalizing Normalization method setup (steps 9–13) | ||
9 | Define Remaining Labware Types | Click the green Normalized Plate box, and then select the labware from the list. The Maximum Volume (µL per well or channel) for the selected plate is automatically displayed. Note: The final normalized sample volume must be equal to or less than the well volume of the selected plate type, or the Maximum Volume (µL per well or channel) cell will turn red. Click the green Diluent Reservoir box, and then select the labware from the list. |
10 | Select the Number of Mix Cycles | Click the green mix cycles box, and then select the value from the list. Note: The number of mix cycles is dependent on the volume, viscosity, and size of molecules in the solution being mixed. |
11 | Input Evaporation Correction Factor | Type a value in the green (%) box for the correction factor, and then click Apply Correction Factor. The setup tool automatically adjusts the values in the Final Diluent Volumes and Final Sample Volumes plate grids. |
12 | Check Volume Required for Diluent Reservoir | Review the Diluent required for Diluent Reservoir area at the bottom of the worksheet. If a red highlight appears on the Total to fill Reservoir value, the diluent volume plus required overage is more than the selected labware can hold. In this case, select a different labware option for the Diluent Reservoir in step 9. Make a note of the Total to fill Reservoir volume. You will use this value to prepare the diluent reservoir for the Normalization run. This volume includes the required overage. |
13 | Create a Normalization Method File | Click Create Method File. After you save the method, the Method Setup Tool closes. When you select the method in the Normalization utility, the form displays the corresponding labware selections and diluent preparation instructions. Follow the instructions that appear in the form to prepare the diluent and sample plates. |
Step 1) Import Concentrations
To import the values for the known concentrations into the initial samples plate, use one of the following procedures:
Note: Empty cells or cells with a value of 0 are acceptable.
Importing values from an Excel file
To import the values from an Excel file (.xls or .xlsx):
1 To clear any data from the Normalization Method Setup Tool, click Clear All (upper right corner).
2 Click Import Sample Concentrations. The Import Initial Sample Concentrations dialog box opens.
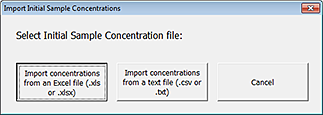 |
3 Click Import concentrations from an Excel file (.xls or .xlsx).
4 In the Open Excel File dialog box, select the file, and then click Open.
The selected Excel file opens and the Select Data dialog box opens.
5 In the Excel file, select the 8-row by 12-column array of values to be imported, and then click OK in the Select Data dialog box.
Note: When selecting data from an Excel sheet, the selected range must be an 8-row by 12-column array of values that correspond to the well positions of the samples.
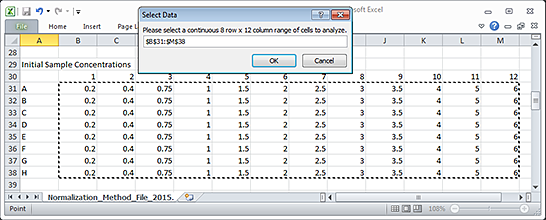 |
The Excel file closes and the imported values appear in the Initial Sample Concentrations area.
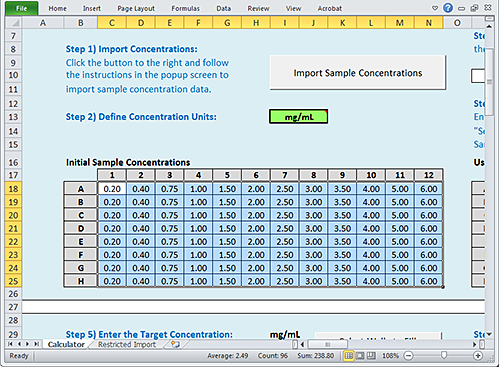 |
Importing values from a text file
To import the values from a text file (.csv or .txt):
1 Click Import Sample Concentrations. The Import Initial Sample Concentrations dialog box opens.
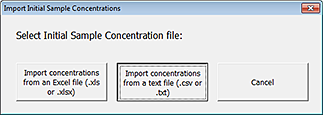 |
2 Click Import concentrations from a text file (.csv or .txt).
3 In the Import Text File dialog box, select the file and click Import.
4 In the Excel Text Import Wizard that appears, follow the instructions to specify the type of delimited file.
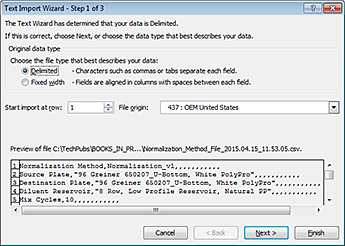 |
5 In the Import Data dialog box, select Existing worksheet, and then click OK.
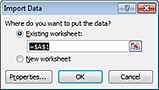 |
The content of the imported file appears in the Restricted Import worksheet of the Method Setup Tool, and the Select Data dialog box opens.
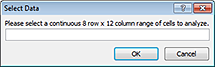 |
6 In the imported worksheet, select the 8‑by‑12 array of values, and then click OK in the Select Data dialog box.
The selected values appear in the Initial Sample Concentrations area.
 |
Step 2) Define Concentration Units
To define the concentration units:
In the green box next to Step 2) Define Concentration Units, verify that the unit of measure for the sample concentration is correct. If necessary, type the correct units.
 |
This input is only a reminder. It has no impact on the subsequent data or volume calculations.
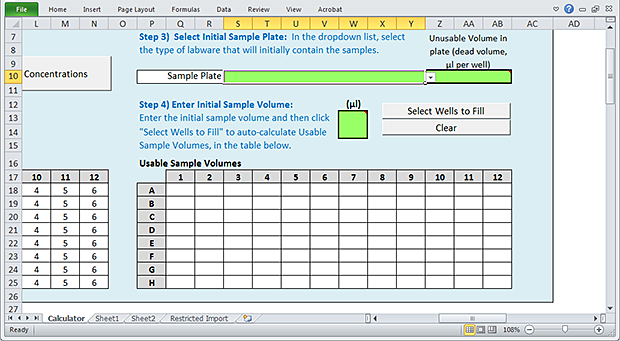
Step 3) Select Initial Sample Plate
To select the initial Sample plate:
1 Click the Sample Plate box, and then select the labware from the list.
 |
2 Notice the value that automatically appears in the Unusable Volume box. To calculate the usable volume, the calculator will subtract this value from the value in Step 4) Initial Sample Volume.
The unusable volume (also known as dead volume) is the volume that cannot be reliably aspirated from the microplate well due to pipetting limitations that arise from differences in well-bottom geometry.
The default value for Unusable Volume is a recommended setting that is based on extensive testing with the labware. If necessary, you may change the value, for example if the value is not appropriate for the sample liquid.
For a list of recommended values for each allowable labware type, see Labware.
Note: The Labware Reference Guide also presents labware-specific pipetting overage and maximum well capacity. You can find this guide in the Literature Library page of the Protein Sample Prep Workbench.
Step 4) Enter Initial Sample Volume
Use the following procedure to define a volume of sample that is initially present in each well of the Sample plate.
To enter the initial Sample volume:
1 In the green (µL) box, type the initial sample volume.
2 Click Select Wells to Fill.
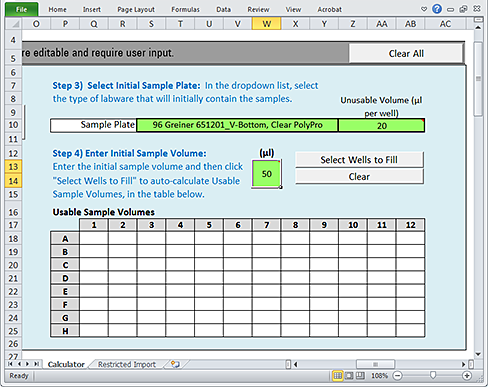 |
3 When the Initial Sample Volume dialog box opens:
a Select the array of wells to be filled in the Usable Sample Volumes plate grid of the worksheet.
b Click OK in the Initial Sample Volume dialog box to populate the selected wells of the plate grid.
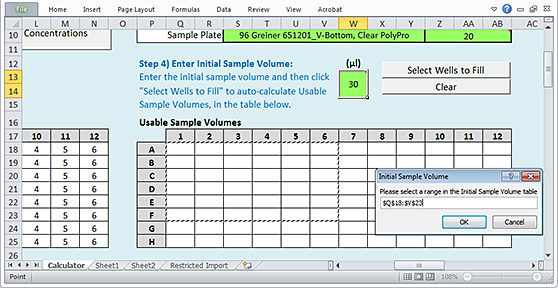 |
Note: The values that populate the plate grid represent the volume of the Initial Sample Volume that is usable. This value is the difference between the value you entered in the green box for Initial Sample Volume and the value in the Step 3) Unusable Volume box.
If you want to clear all values from the Usable Sample Volumes plate grid, click Clear.
Step 5) Enter the Target Concentrations
Use the following procedure to specify the desired concentrations after the samples are normalized.
To enter the target concentrations:
1 In the green box, type the concentration.
2 Click Select Wells to Fill.
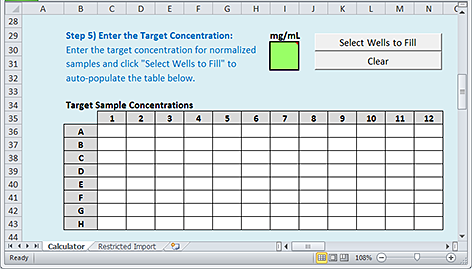 |
3 When the Target Sample Concentration dialog box opens:
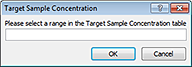 |
a Select the array of wells to be filled in the Target Sample Concentrations plate grid of the worksheet.
b Click OK in the Target Sample Concentration dialog box to populate the selected wells of the plate grid.
Note: If you want to clear all values from the Target Sample Concentrations plate grid, click Clear.
Step 6) Enter the Target Volume
Use the following procedure to specify the desired volumes after the samples are normalized.
To enter the target volume:
1 In the green (µL) box, type a value that you want to apply to multiple wells of the microplate.
2 Click Select Wells to Fill.
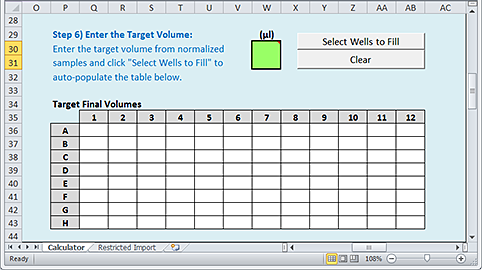 |
3 When the Target Final Volume dialog box appears:
 |
a Select the array of wells to be filled in the Target Final Volumes plate grid of the worksheet.
b Click OK in the Target Final Volume dialog box to populate the selected wells in the plate grid.
Note: If you want to clear all values from the Target Final Volumes plate grid, click Clear.
Note: If the next step in the workflow involves an AssayMAP application, all the samples should be at the same target volume.
Step 7) Calculate Normalization Volumes
To calculate the normalization volumes:
Click Calculate Volumes. An algorithm uses the information provided in the previous steps to calculate the sample and diluent volumes required to achieve the specified normalization targets. The calculated volumes (µL) are displayed in the following areas:
• Diluent Volumes to Use plate grid
• Sample Volumes to Use plate grid
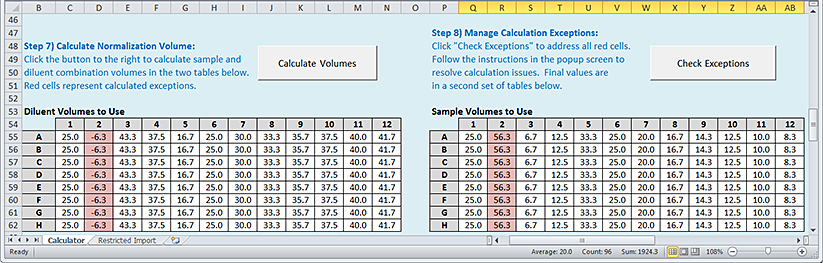 |
Any wells with red highlights are considered exceptions, for which the calculated sample or diluent volume values cannot be achieved because the volumes required are either larger than the volumes available in the Sample plate or smaller than practical for the AssayMAP Bravo Platform to transfer with high accuracy.
Step 8) Manage Calculation Exceptions
In this step, you decide how to address each of the exceptions that are indicated by red highlighted wells in the plate grid.
To manage the exceptions:
1 Click Check Exceptions. The Manage Exceptions dialog box opens. The descriptions change in the Status and the Selecting Do Best Will areas depending on the conditions that caused the exception for the selected sample. The following figure shows an example.
Figure Example of the Manage Exceptions dialog box
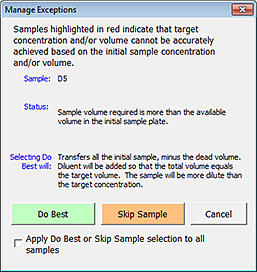 |
2 Select the option that is appropriate for your samples. The options are as follows:
Option | Description |
|---|---|
Do Best | The resulting actions for using this option depend on the conditions that caused the exception. An explanation of the action for the selected sample is displayed in the Selecting Do Best Will area. For descriptions by the type of exception, see the following section, Do Best option and types of exceptions. In general, Do Best adjusts the calculated sample and diluent volumes to get as close to the target concentration as possible while maintaining the target volume. The tool gives a higher priority to the target volume than the target concentration, assuming that the Normalized plate will be used in another AssayMAP Bravo run, and the well volume differences across the plate can lead to unpredictable assay results. The values from the calculations appear in the Expected Concentrations after Normalization plate grid at the bottom of the worksheet. You may apply Do Best in the following ways: • Click Do Best for each exception, sample by sample. • Select Apply Do Best or Skip Sample selection to all samples, and then click Do Best. The action is performed on all the remaining exceptions. After addressing all the exceptions, click Close to close the dialog box. |
Skip Sample | This option skips the normalization process for the selected sample. If selected, nothing will be transferred into the Normalized plate at the specified well location. You may apply Skip Sample in the following ways: • Click Skip Sample for each exception, sample by sample. • Select Apply Do Best or Skip Sample selection to all samples, and then click Skip Sample. The action is performed on all the remaining exceptions. After addressing all the exceptions, click Close to close the dialog box. Note: No pipette tip is used for blank wells or skipped wells. |
Cancel | Click Cancel to exit the Manage Exceptions dialog box if: • No exceptions remain and you want to move on to the final steps of defining the normalization method. • You want to return to an earlier step in the method setup process to make changes. |
Apply Do Best or Skip Sample selection to all samples | The option to perform the Do Best or Skip Sample action on all the remaining wells. Select the Apply Do Best or Skip Sample selection to all samples check box, and then click Do Best or Skip Sample, as applicable. After addressing all the exceptions, click Close to close the dialog box. |
Do Best option and types of exceptions
If you select the Do Best option, the resulting Bravo actions vary depending on the conditions that caused the exception. The following four conditions cause exceptions:
1 The initial concentration value for a sample is lower than the target concentration for that sample.
2 The usable sample volume value is insufficient to cover the volume required to meet the normalization target concentration or target volume.
3 The calculated sample volume is less than 5 µL, which is the minimum practical volume at which high accuracy and precision (5% CV, 10% Relative Inaccuracy) can be achieved when using the AssayMAP Bravo Platform.
4 The initial concentration for a sample is lower than the target concentration for that sample, and the usable volume in the initial Sample plate is not sufficient to allow for the entire sample to be transferred directly into the Normalized plate.
The following table describes the status messages and Do Best behaviors for each of the four exceptions.
Exception | Status message | Do Best behavior |
|---|---|---|
1 | Initial concentration is below the target concentration. | No dilution will take place. Only sample will be transferred to the Normalized plate, which will cause the sample to have a concentration that is lower than the specified target concentration. |
2 | Sample volume required is more than is available in the Initial Sample plate. | The deficit in sample volume will be substituted with diluent, causing the concentration to be lower than expected, but ensure that the target volume is still met. |
3 | Sample volume required is less than 5 µL. Five microliters is the minimum volume that can be transferred with a high degree of accuracy. | The AssayMAP Bravo Platform will attempt to perform the normalization as defined, by pipetting less than 5 µL of sample. |
4 | Initial concentration is below the target concentration and the initial volume is less than the target volume. | The deficit in sample volume will be substituted with diluent, causing the concentration to be lower than expected, but ensure that the target volume is still met. |
As the exceptions are managed:
• If Do Best was selected, the new values calculated for the diluent and sample volume are highlighted in the Final Diluent Volume and Final Sample Volume plate grids.
• If Skip Sample was selected, the word “Skip” appears as the value for both the Final Diluent Volume and the Final Sample Volume.
Step 9) Define Remaining Labware Types
To specify labware for the remaining deck locations:
1 Click the Normalized Plate green box, and then select the labware from the list.
Note: This plate will be placed on a Bravo Plate Riser at deck location 6, which is where the final normalized samples will be at the end of the run.
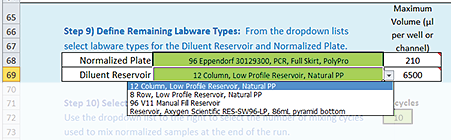 |
2 Notice the value that automatically displays in the Maximum Volume (µL per well or channel) box.
3 Click the Diluent Reservoir green box, and then select the labware from the list.
Note: This reservoir will be placed at deck location 4 without a Bravo Plate Riser. The reservoir will supply diluent for the normalization process.
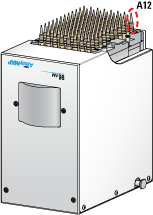
4 Notice the corresponding value in the Maximum Volume (µL per well or channel) box. This value minus the dead volume associated with the selected plate type is the volume that the Bravo 96AM Head can access in the selected labware. The Bravo 96AM Head uses a single pipette tip mounted on probe A12 to aspirate the volume.
For example, in a 12‑column reservoir, the accessible volume is only the rightmost column, and in an 8‑row reservoir, the accessible volume is only the topmost row.
Figure Upside down view of AssayMAP head showing probe A12
 |
Step 10) Select the Number of Mix Cycles
In this step you select the number of mix cycles to perform after all the samples and diluent are added in the Normalized plate. The Bravo 96AM Head presses on the full selection of used pipette tips from deck location 3, and then mixes all the wells at the same time.
The mixing volume is 75% of the cumulative plate volume, up to the maximum capacity of the pipetting method, where
Capacity for 250 µL pipette tips = 140 µL
Normalization methods for large volumes may require a greater number of mix cycles, which you should determine empirically.
To select the number of mix cycles:
Click the mix cycles box, and then select the value from the list.
 |
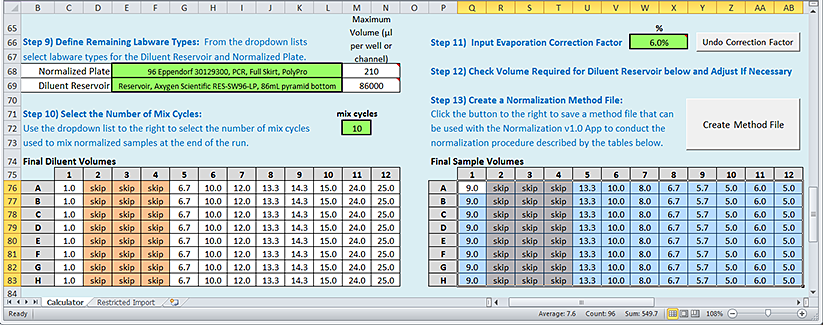
Step 11) Input Evaporation Correction Factor
In general, a Normalization protocol run of 96 samples on the AssayMAP Bravo Platform requires approximately 110 minutes. During this time, the samples can evaporate, especially by the time the 96th sample is transferred. The optional Evaporation Correction Factor helps to compensate for the sample evaporation.
The evaporation rate will vary depending on the sample solution. You should empirically determine the correction factor using test samples. See Assay development guidelines for a detailed explanation of when to use this factor and how to calculate an appropriate value to use.
To account for sample evaporation:
1 In the Step 11) green box, type a value (%) for the correction factor, and then click Apply Correction Factor.
The tool automatically adjusts the values in the Final Diluent Volumes and Final Sample Volumes plate grids. The button label changes to Undo Correction Factor.
Note: If you want to undo the change, click Undo Correction Factor.
 |
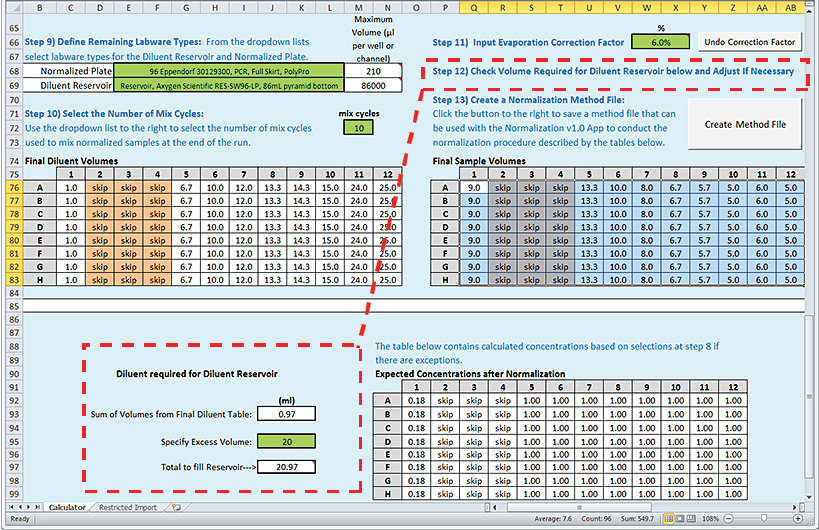
Step 12) Check Volume Required for Diluent Reservoir below and Adjust If Necessary
To verify that the Diluent reservoir has sufficient volume:
1 Review the Diluent required for Diluent Reservoir area at the bottom of the worksheet. The Method Setup Tool calculates the total fill volume for the Diluent reservoir based on the calculated Final Diluent Volumes and the recommended excess volume for the selected labware type.
Note: For a list of recommended values for each allowable labware type, see Labware.
2 If a red highlight appears on the Total to fill Reservoir value, the diluent volume required is more than the selected labware can hold. In this case, select a different labware option for the Diluent Reservoir in Step 9).
Note: If you are using a 12-column reservoir or an 8-row reservoir, the Total to fill Reservoir value represents the volume in the rightmost column or topmost row, respectively.
3 Make a note of the Total to fill Reservoir volume. You will use this value to prepare the diluent reservoir for the normalization run.
 |
Step 13) Create a Normalization Method File
Note: If you manually type the values into the plate grids of the Normalization Method Setup Tool, you may have unexpected results.
To create the Normalization method file:
1 Click  .
.
 .
.2 In the login window, type your VWorks user name and password, and click Log In.
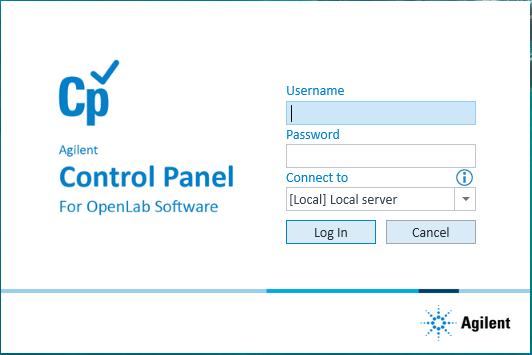 |
3 In the Save File dialog box, specify the file name and the storage location, and then click Save.
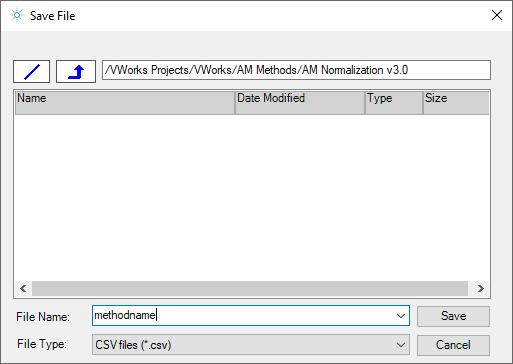 |
• VWorks Plus default storage location:
… /VWorks Projects/VWorks/AM Methods/AM Normalization v3.0
… /VWorks Projects/VWorks/AM Methods/AM Normalization v3.0
• VWorks Standard default storage location:
C:\OLSS Projects\VWorks Projects\VWorks\AM Methods\AM Normalization v3.0
C:\OLSS Projects\VWorks Projects\VWorks\AM Methods\AM Normalization v3.0
Note: The method file is stored as a record of interest (ROI) in an archive (.csv.roiZip). VWorks cannot load files (.roiZip extension) that have been modified or renamed outside of the Protein Sample Prep Workbench or VWorks software.
4 Click OK when the uploaded successfully message appears. The Method Setup Tool closes.
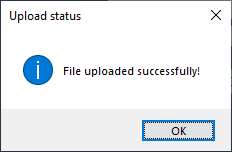 |
Next steps:
If you want to view the method in Microsoft Excel, see Exporting and importing AssayMAP methods
If you are ready to prepare the serial dilution plates, see Preparing the diluent and samples.