Running the protocol

The Phosphopeptide Enrichment protocol:
• Washes the syringes.
• Primes and equilibrates the cartridges to prepare for sample loading.
• Loads the samples onto the cartridges.
• Removes non-specific binding molecules from the cartridges.
• Elutes the peptides from the cartridges.
For some of these operations the cartridges are mounted on the syringe probes, while for other operations the cartridges are parked in the cartridge seating station.
Experiment ID and method requirements
Each workbench application and utility has an Experiment Settings section that allows you to select an experiment ID and a method.
• An experiment ID is a database record that captures the steps executed and the settings used during each run of an application or utility. Any errors that may have occurred during a run are also recorded.
To create an experiment ID, you open the Experiments Editor by clicking  in any Workbench app or utility. For details, go to the Literature Library and open Using the Protein Sample Prep Workbench. In the browser that opens, click Using Experiment IDs.
in any Workbench app or utility. For details, go to the Literature Library and open Using the Protein Sample Prep Workbench. In the browser that opens, click Using Experiment IDs.
 in any Workbench app or utility. For details, go to the Literature Library and open Using the Protein Sample Prep Workbench. In the browser that opens, click Using Experiment IDs.
in any Workbench app or utility. For details, go to the Literature Library and open Using the Protein Sample Prep Workbench. In the browser that opens, click Using Experiment IDs.• A method is a comprehensive collection of saved settings for an application or utility, which you can use to run the application or utility.
Experiment IDs and methods are required for compliance-enabled VWorks editions and optional for noncompliance-enabled VWorks editions.
VWorks edition | Experiment ID and method selection |
|---|---|
VWorks Plus | Required |
VWorks Standard | Optional |
Before you start
Ensure that you:
• Prepare the reagents. See Preparing the solutions.
• Prepare the samples. See Preparing the samples.
• If applicable, make sure that you know which experiment ID to use to record the steps executed during the utility and app runs.
• Run the Startup protocol to prepare the AssayMAP Bravo Platform for the run. See the System Startup/Shutdown v3.0 User Guide utility.
• Transfer the cartridges to the Cartridge & Tip Seating Station. See Cartridge Transfer v2.0 User Guide.
Setting up the protocol
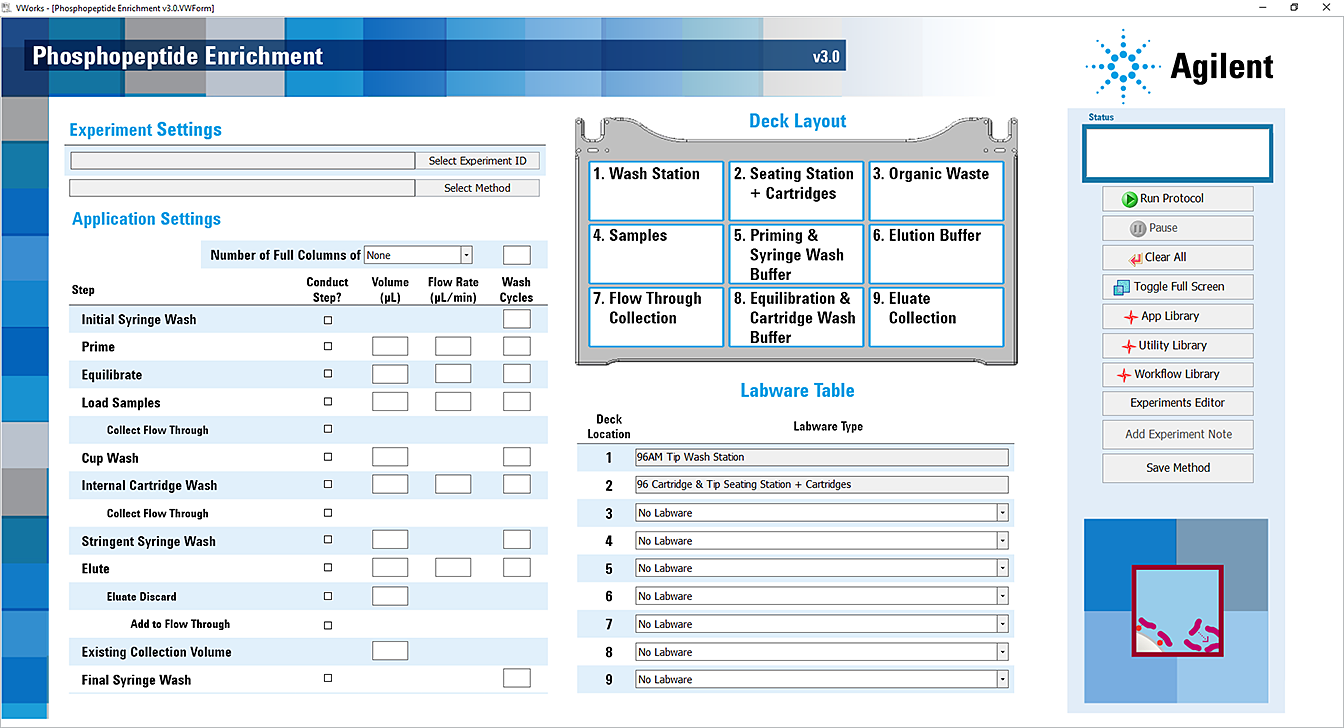
Before starting the protocol, make sure the appropriate selections and values are specified in the Phosphopeptide Enrichment application.
To set up the protocol:
1 Open the App library.
2 Locate Phosphopeptide Enrichment, and then click App.
 |
The Phosphopeptide Enrichment application opens.
 |
3 If applicable, click Select Experiment ID.
 |
The Experiments Editor opens.
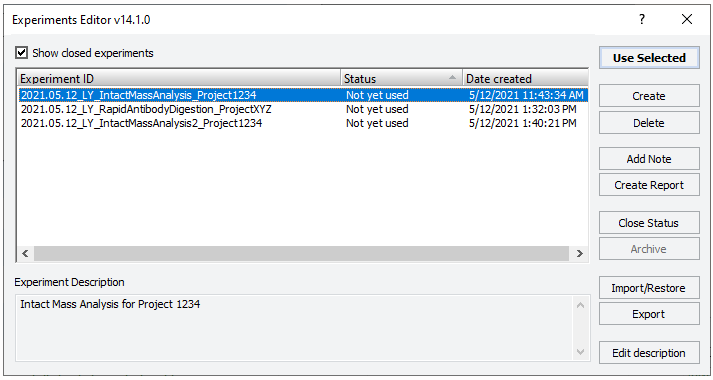 |
4 Select the Experiment ID that you want to use to record the steps performed during this application run, and then click Use Selected.
The Experiments Editor closes.
5 In the form, click Select Method to locate and select a method.
In the Open File dialog box, select the method, and click Open.
• To run the selected method, go to Starting the protocol run.
• To modify the method or create a method, proceed to step 6.
VWorks Plus. Administrator or technician privileges are required to create and modify methods.
6 In the Application Settings area, specify the cartridge settings:
 |
a Select the cartridge size from the list:
• 5 µL Cartridges
• 25 µL Cartridges
b In the box, type the number of full columns of cartridges to be used.
The position of the columns of cartridges in the tip seating station must match the positions of the samples and solutions in the plates on the deck.
Range: 1–12
Default: 1
7 Under Application Settings, select the check boxes of the steps that you want to perform, and enter the values for the selected steps.
Note: For any unselected steps, ensure that the volume, flow rate, and wash cycles boxes are blank to avoid potential confusion when a experimental report is generated.
8 In the Labware Table area, select the labware you are using for the protocol run.
Note: If all the steps that use a certain labware location are unchecked, ensure that the labware selection is No labware to avoid confusion when setting up the deck and when generating an experimental report. The Reagent volume calculator is a good resource for this decision because it returns a value of zero in the Volume per well required cell if no labware is needed.
9 To save the method:
a Click  .
.
 .
.b In the Save File As dialog box, type the file name and click Save.
Note: Agilent recommends that you use the cartridge size (5 µL or 25 µL) as a prefix to the name.
VWorks Plus. You must save the method before you can run it.
Application Settings
The following table gives a brief description of each setting. For details, including the practical ranges of values for a given setting, see the Assay development guidelines and protocol notes.
Table Application Settings overview
Steps* | Description | Cartridge size | Volume (µL) | Flow Rate (µL/min) | Wash Cycles |
|---|---|---|---|---|---|
Initial Syringe Wash | Washes syringes at the wash station (deck location 1). | 5 µL: | – | – | 3 |
25 µL: | – | – | 3 | ||
Range: | – | – | 0–10 | ||
Prime | Aspirates Priming Buffer (deck location 5) into the syringes, and then dispenses it through the cartridges into the Organic Waste plate (deck location 3). | 5 µL: | 100 | 300 | 1 |
25 µL: | 250 | 300 | 1 | ||
Range: | 0–250 | 0.5–500 | 0–10 | ||
Equilibrate | Aspirates Equilibration Buffer (deck location 8) into the syringes, and then dispenses it through the cartridges into the Organic Waste plate (deck location 3). | 5 µL: | 50 | 10 | 1 |
25 µL: | 250 | 10 | 1 | ||
Range: | 0–250 | 0.5–500 | 0–10 | ||
Load Samples | Aspirates samples (deck location 4) into the syringes, and then dispenses them through the cartridges into the Organic Waste plate (deck location 3) or into the Flow Through Collection plate (deck location 7). | 5 µL: | 100 | 5 | 3 |
25 µL: | 100 | 5 | 3 | ||
Range: | 0–1000 | 0.1–500 | 0–10 | ||
Collect Flow Through | If selected, collects the sample flow-through in the Flow Through Collection plate (deck location 7). If not selected, discards the sample flow-through in the Organic Waste plate (deck location 3). | – | – | – | – |
Cup Wash | Rinses the cartridge cups with Cartridge Wash Buffer (deck location 8), and then discards the liquid into the Organic Waste plate (deck location 3). | 5 µL: | 25 | – | 3 |
25 µL: | 25 | – | 3 | ||
Range: | 0–100 | – | 0–10 | ||
Internal Cartridge Wash | Aspirates Cartridge Wash Buffer (deck location 8) into the syringes, and then dispenses it through the cartridges into the Organic Waste plate (deck location 3) or into the Flow Through Collection plate (deck location 7). | 5 µL: | 50 | 10 | 3 |
25 µL: | 250 | 10 | 3 | ||
Range: | 0–250 | 0.5–500 | 0–10 | ||
Collect Flow Through | If selected, collects the Internal Cartridge Wash flow-through at the Flow Through Collection (deck location 7). If not selected, discards the Internal Cartridge Wash flow-through into the Organic Waste (deck location 3). | – | – | – | – |
Stringent Syringe Wash | Aspirates Syringe Wash Buffer (deck location 5) into the syringes, and then dispenses it into the Organic Waste plate (deck location 3). | 5 µL: | 50 | – | 2 |
25 µL: | 50 | – | 2 | ||
Range: | 0–250 | – | 0–10 | ||
Elute | Aspirates Elution Buffer (deck location 6) into the syringes, and then dispenses it through the cartridges into the Eluate Collection plate (deck location 9). | 5 µL: | 25 | 5 | 1 |
25 µL: | 125 | 5 | 1 | ||
Range: | 0–250 | 0.1–500 | 0–10 | ||
Eluate Discard | If selected, a specified initial volume of the eluate is discarded into the Organic Waste plate (deck location 3), or collected in the Flow Through Collection plate (deck location 7). | 5 µL: | 0 | – | – |
25 µL: | 0 | – | – | ||
Range: | 0–250 | – | – | ||
Add to Flow Through | If selected, collects the Eluate Discard in the Flow Through Collection plate (deck location 7). If not selected, discards the Eluate Discard into the Organic Waste plate (deck location 3). | – | – | – | – |
Existing Collection Volume | Specifies the volume of liquid present in the Eluate Collection plate (deck location 9) at the beginning of the run. | 5 µL: | 0 | – | – |
25 µL: | 0 | – | – | ||
Range: | 0–300 | – | – | ||
Final Syringe Wash | Washes the syringes at the wash station (deck location 1). | 5 µL: | – | – | 3 |
25 µL: | – | – | 3 | ||
Range: | – | – | 0–10 | ||
*For practical value ranges for the steps listed in this table and factors to consider when changing the default values, see the Protocol stepwise guidelines. For a complete list of the robotic movements executed during a run, see Automation movements during the protocol. | |||||
About performing a mock run (optional)
If you are unfamiliar with the protocol and would like to see how it operates before running it with valuable samples and reagents, you can perform a mock run. A mock run uses empty or water-filled labware and source bottles.
You prepare for a mock run the same way you would prepare for a real protocol run, except that you use empty labware for a totally dry run or labware containing water for a wet run. To decrease the run time, you can increase the flow rates to 500 µL/min, change the wash cycles to 1, and decrease the volumes. Use the AssayMAP Resin-Free cartridges instead of packed cartridges for mock runs.
Starting the protocol run
Note: The Greiner and BioRad PCR plates are not compatible with the 25 µL cartridges at deck locations 7 and 9.
To start the protocol run:
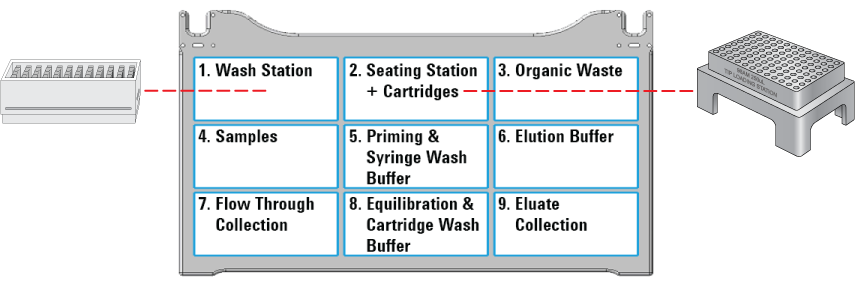
1 Ensure that the accessories, filled reagent plates, and collection plates are at the assigned deck locations, as shown in the Deck Layout image of the form.
Make sure the labware are properly seated on the Bravo deck.
 |
2 Click  to start the run.
to start the run.
 to start the run.
to start the run. To monitor the progress of the run, check the Status box.
 |
After the protocol run starts, you can walk away from the AssayMAP Bravo Platform for the duration of the protocol. The default protocol with 100‑µL samples and 5-µL/min sample loading flow rate should take approximately 50 minutes to complete.
To pause the run, click Pause. The task currently in progress finishes before the protocol pauses. The Scheduler Paused dialog box opens. For details, see Emergency stops and pauses.
To troubleshoot errors, see the Error Recovery Guide and the Bravo Platform User Guide in the Literature Library page of the Protein Sample Prep Workbench.
Adding an experiment ID note after the run
After the protocol run ends or during a pause, you can add a note to the experiment ID. For example, a note can describe any observations during the run or any offline steps that are being executed. The notes that you add will appear in any reports generated for the experiment ID.
To add a note to an open experiment ID:
1 While the experiment ID is still selected in the Experiment Settings area, click  . The Add Note dialog box opens.
. The Add Note dialog box opens.
 . The Add Note dialog box opens.
. The Add Note dialog box opens.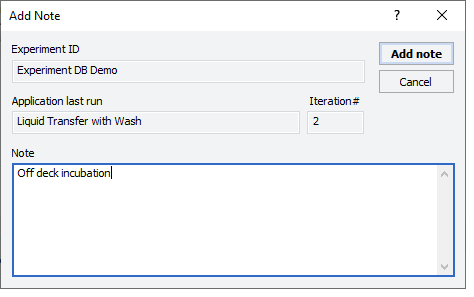 |
2 In the Note area, type the note, and then click OK.
For detailed instructions on working with Experiment IDs, see Using Experiment IDs.
Cleaning up
To clean up after a run:
1 Remove used labware from the deck.
2 Discard leftover reagents appropriately.
3 Optional. Conduct stringent washing of the syringes:
a Open the Syringe Wash utility  .
.
 .
.b If applicable, click Select Experiment ID to open the Experiments Editor.
 |
c In the Experiments Editor, select the Experiment ID that you want to use to capture the steps performed during this utility run, and then click Use Selected.
d Click Select Method to select and load the method for this utility.
e Confirm that the labware and accessories on the AssayMAP Bravo deck match the display in the Deck Layout area of the form.
f Click  to start the run.
to start the run.
 to start the run.
to start the run.To shut down at the end of the day:
Run the System Shutdown utility. See System Startup/Shutdown v3.0 User Guide.